Evolution is a master recycler. We often use old structures (or ancient genes) for new work. Mammalian ears are a good example. Over time, the jawbone of our fish ancestors evolved into three separate small bones that transmit sound waves from the eardrum to the inner ear.
New research shows there was another hand-me-down from fish to mammals. It turns out that the flexible cartilage of fish gills closely corresponds to the cartilage of the external ear (the visible part of the ear) of mammals. Indeed, flexible cartilaginous structures play different roles in fish and mammals. The gill structure allows fish to breathe, and the cartilage in the mammal’s outer ear captures sound. However, the underlying genetic networks that build these structures share a common history.
To be clear, the gill structure did not transform into the external ear of mammals. Rather, when the first vertebrates appeared on land and lost their gills, the underlying genetic network that forms gill cartilage was able to build something new. “It’s one of the surprises of life and evolution,” says Abigail Tucker, professor of development and evolution at King’s College London, who was not involved in the study. “The regulatory network was still present, so we were able to capture and reuse it, this time to create the structure of the outer ear rather than the gills.”
About supporting science journalism
If you enjoyed this article, please consider supporting our award-winning journalism. Currently subscribing. By subscribing, you help ensure future generations of impactful stories about the discoveries and ideas that shape the world today.
This recycling of the same gene network provided the basis for subsequent evolutionary innovations. The cartilage in the outer ears of mammals has further evolved into a variety of shapes, including the large, sensitive ears of echolocating bats, the pointy ears of alert cats, and the droopy ears of elephants. Sounds that are important to the animal. In some mammals, ear cartilage is further modified and is filled with specialized chondrocytes containing large lipid droplets, which give the cartilage unique structural and acoustic properties, the researchers say. I’m making a hypothesis.
“We learned from our ancestors who built cartilage-filled gills in their heads, which moved into a position more closely associated with the ears during evolution, just as the jawbones of our fish ancestors moved into the middle ear. We believe there is a program,” Gage Crump explains. He is a stem cell biologist at the University of Southern California and the lead author of a new study published in 2006. nature. “Although the program for growing cartilage structures in this general region of the head is deeply conserved, the exact location, the complete repertoire of expressed genes, and therefore the cell types and their functions vary considerably.”
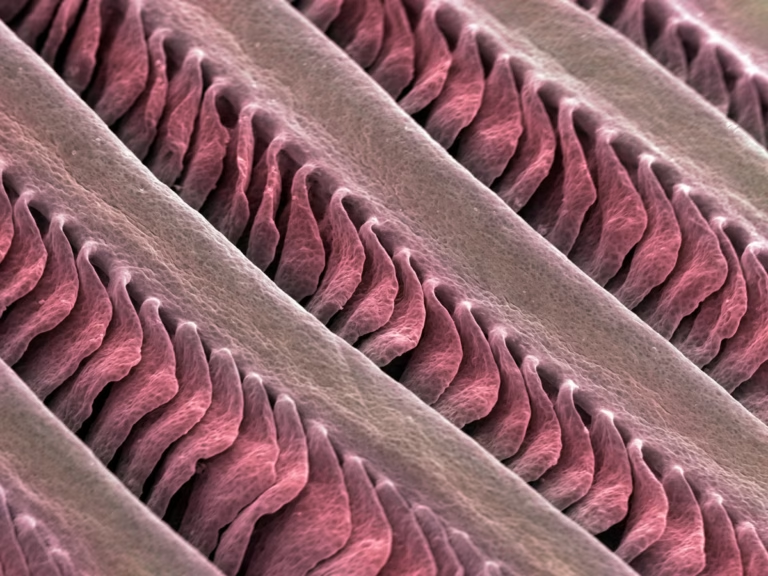
Crump’s team, which uses zebrafish as a model organism for their research, has long been interested in facial development in vertebrates. When researchers created an atlas of all the different cell types in the zebrafish face, they noticed two types of cartilage. One was expected and the other was something I hadn’t noticed before. This unexpected shape was a rod of elastic cartilage that supported finger-like projections on the gills. The cartilage was similar to the type found in the outer ear of mammals.
The researchers observed that gene activity in human external ear cartilage was similar to gene activity in the elastic cartilage of fish gills. However, many genes are also active in unrelated organs. To see whether these structures share an evolutionary history, the researchers focused on enhancers, DNA sequences that drive the activity of target genes in specific tissues. They identified six important enhancers that are essential for cartilage development in the human outer ear but not in the nose. The researchers reasoned that if gene activity in fish gills and mammalian ear cartilage is initiated by similar enhancers, these structures likely share the same evolutionary origin.
This approach, which focuses on enhancers, is “very exciting, really very intelligent, very insightful,” says the University of California, San Francisco’s Stem Cell and Development Professor, who was not involved in the study. says biologist Licia Sellari. “This could reveal whether new structures arose from the exploitation of ancestral developmental programs or whether they emerged de novo.”
To investigate the question of gill ears in fish, researchers led by Crump’s then-graduate student Mati Thirupathy performed several sophisticated gene transfer experiments. First, they engineered six human external ear enhancer genes into the zebrafish genome and used fluorescent reporter genes to pinpoint the locations in the body where the enhancers’ normal targets are activated. Surprisingly, the human ear cartilage enhancer only promotes green fluorescent protein activity in the zebrafish gills, and whatever controls gene expression is very similar in the gills and external ear. This suggests that, Crump said.
So the team conducted another experiment. A key enhancer that is active in zebrafish gills was incorporated into the mouse genome. There, the researchers observed that fish DNA elements activated green fluorescent protein in the outer ears of developing transgenic mice, suggesting that the same underlying gene network is used to build gill and ear cartilage. It reinforced the idea that there is.
“What’s more interesting than just reusing the same molecular toolkit is the fact that we’re also reusing the regulatory elements (enhancers) that control the expression of those genes.” Professor Tucker said the gills are the mammalian ear. It is said to promote the expression of cartilage genes. “So the level of use of the system that was already there has increased even further.”
The researchers then sought to identify which important genes are under the influence of these enhancers. One gene family that stands out is DLX, This is related to a gene identified in Drosophila. distal free It is important for insect limb development. Researchers discovered that the same enhancer exists in vertebrates. DLX Genes have appeared in animals over 400 million years of evolution, from zebrafish to humans. That’s why they were able to replace the enhancer with genetically engineered fish and mice.
To find out how old these enhancers are, the researchers looked at horseshoe crabs, which are also gill-breathing invertebrates. they discovered the same thing distal free genes related to DLX This gene is also involved in the formation of horseshoe crab gills. By inserting horseshoe crab DNA regulatory elements into the zebrafish genome, they were able to activate fluorescent molecules in the zebrafish’s gills. This suggests that the genetic machinery that creates the mammalian outer ear is older than vertebrate evolution. Its origins may be traced back to some of the first marine invertebrates to have gill-like projections. When the first vertebrates, fish, evolved, the gene networks that build gill cartilage from those invertebrates were recycled to make fish gills, even though fish evolved a new type of bony skeleton. I did.
“We think that the elastic cartilage in our outer ear may be the last remnant of invertebrate cartilage,” Crump speculates.
To understand what happened along the vertebrate evolutionary tree between fish and mammals, the researchers examined the activity of the same enhancers in frogs and lizards. In frog tadpoles, the human external ear enhancer activated fluorescent proteins in the tadpole gills. In anole lizards, which have neither gills nor external ears, the human external ear enhancer activated fluorescent proteins in the animals’ ear canals. The ear canal also contains elastic cartilage similar to the gills of fish and tadpoles. This suggests that the gene network that makes the elastic cartilage in fish gills was activated first in the reptile ear canal and then in the mammalian ear.
“So what we imagine is that there was a transition from gills to external auditory canal in amphibians and reptiles, followed by extensive elaboration in mammals to form the external ear.” Crump says.
During the course of evolution, mammalian external ear cartilage continued to evolve not only in shape but also in internal composition. Maxim Plikas, a cell biologist at the University of California, Irvine, and his team recently discovered that the cartilage cells in the ears of small mammals (especially mice, shrews, bats, and rats) are a type of chondrocyte and fat cell. Reported. Filled with fat droplets, these cells form a bubble-wrap-like tissue called fatty cartilage. This tissue was first discovered in 1854 by German histologist Franz von Leydig, but has been largely forgotten until now. Plikus’ team hypothesizes that fatty cartilage has unique acoustic properties, such as its ability to enhance the propagation of sound waves, which may be adapted for hearing in mammals.
“Certainly there are programs that exist in invertebrates that are reused to form the external ear in fish and mammals, but there are also innovations that appear in mammals,” Sellari says. science About research on fat cartilage. “One of these innovations is the presence of fatty cartilage.”
“[Fatty cartilage]can use the vacuole, or lipid droplet, for a completely different purpose than what is normally thought of,” says Plikas. The main purpose of lipid droplets in adipocytes is to store energy, whereas in adipose cartilage “these lipid droplets play a primarily structural and biomechanical role and no longer contribute to metabolic function.” says Prix.
Postdoctoral researcher Raul Ramos led the research. science. The researchers showed that in mice, fat vacuoles do not change depending on metabolic status. That is, fat vacuole size does not increase when mice are overfed, and fat droplets are not used for energy if the animal starves. The researchers further found that the droplets are created using a very specific metabolic pathway that converts sugar into fat, and that this controlled metabolic pathway allows the animal’s body to regulate the precise size and spacing of the fat droplets. showed.
This has allowed for the evolution of ear structures with acoustic properties suited to the needs of different types of animals. For example, bats’ large, raised ears are sensitive enough to detect the flapping of tiny insect wings.